Administering the COVID-19 Vaccine in the Maritime Industry
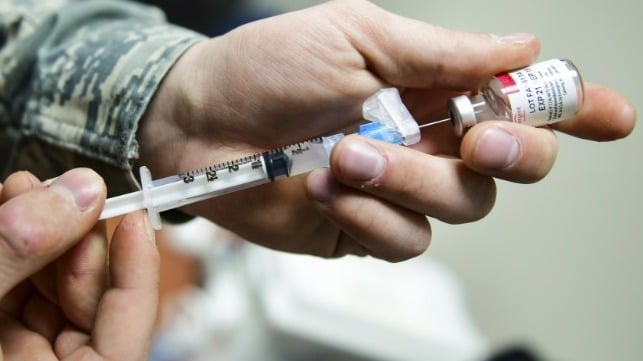
As two historic vaccines undergo distribution to slow the spread of COVID-19, the maritime industry must confront the unique operational obstacles we face in administering them. Our sector is a vital pillar of the global economy. Our mariners are essential to supply chains in critical products like food and energy. Good work is already being done to ensure our workforce is included in Phase 1B and 1C. As we advocate for appropriate classification in the tiered phases of distribution, we must also carefully consider the logistical details involved with handling and administering these storage-sensitive vaccines.
Pfizer and Moderna are the pharmaceutical companies manufacturing the two COVID-19 vaccines available as of early 2021. Both immunizations require two doses of the vaccine. The second dose of Pfizer’s medicine should be administered 21 days after the first dose. The second dose of Moderna’s should be administered 28 days after the first dose. We are learning more about the flexibility and timing of the second dose.
“This is a significant operational challenge,” explains Dr. Ann Jarris, CEO of Discovery Health MD, a medical risk management firm based in Seattle. “You have to schedule people carefully, they have to show up for their appointments, it has to be done on that day, and you have a very narrow window during which you can have the vaccine ready to be given. There is no room for slop.”
If you are administering the first shot, you must be prepared to commit to giving the second shot. This may necessitate either pulling your vessel into port just weeks after your last departure. Storing or administering the vaccine on board is not currently recommended due to the possibility of temperature excursions, the need to protect vaccines from vibration and the limitations of treating significant reactions at sea. Developing an understanding with your local port medical providers on vaccine administration will be critical. States are recommending the first and second dose be given at the same location, but that may not be logistically possible.
Dr. Jarris emphasizes that both vaccines appear to be very safe. Common reactions include soreness or rash at the injection site, fatigue, fever and muscle aches. These may start immediately after the vaccine or become apparent a few days later. Allergic reactions have been reported and appear to be caused by other ingredients found in the vaccines, potentially polyethylene glycol. That being said, individuals with a history of allergic reactions should be observed for 30 minutes after the shot. Individuals without such a history should be observed for 15 minutes. The CDC has released an app called “V-safe” for people to report reactions after vaccination.
Two more pharmaceutical companies, Johnson & Johnson and AstraZeneca, are in the process of developing other COVID-19 immunizations but they aren’t expected to be widely available until later in 2021. These may come in single-dose formulations. Some maritime outfits may choose to wait for that option, but some may need the security and peace of mind that come with a fully immunized workforce as soon as possible. If this is the case, start considering the logistics of your crew’s immunization schedule and work with your local medical providers to coordinate vaccine availability and appointments.

that matters most
Get the latest maritime news delivered to your inbox daily.
Discovery Health MD is a medical risk management firm that specializes in consulting for the maritime industry. They are registered providers of both COVID-19 vaccines and have been working to immunize Phase 1A recipients. If your operation includes Phase 1A workers and you are interested in utilizing Discovery Health MD’s services to coordinate vaccination administration in the Seattle area, write to [email protected] to start the conversation.
The opinions expressed herein are the author's and not necessarily those of The Maritime Executive.
